Abstract
Introduction:High-dose Melphalan followed by autologous hematopoietic cell transplantation (ASCT) compared to conventional chemotherapies has been shown to improve survival and progression free survival (PFS) in eligible patients with multiple myeloma (MM). However cells that are resistant to the high-dose therapy and remaining in the patient and/or PBSC grafts may eventually lead to relapse. Over the past decade, data have been published on the role of circulating plasma cells (CPCs) as a poor prognostic feature at the time of diagnosis and prior to auto HSCT. But these studies have not sought this issue exclusively in PBSC grafts. The aim of this study was to analyze the impact of flow cytometric measurement of residual clonal cells within the apheresis products on outcome following ASCT.
Materials and Methods:Patients with a diagnosis of MM who underwent auto HSCT at our center between January 2008- Mart 2018 were prospectively analyzed. PBSC grafts were tested for the presence of abnormal PCs (APC) and the number of normal PCs (NPC) by multi-parameter flow cytometry (FCM). Standard panel was set up with CD138FITC/CD38PE/CD45ECD/CD56PC5, CD45-CD56+PCs were identified as APC if any aberrant expression (including; CD20(loss)CD27(loss)CD28(gain)CD33(loss)CD34(gain)CD81(loss) CD117(gain)) is detected at diagnosis, the corresponding antibody was also added to the panel. Since maintenance was not an approved treatment in myeloma most patients did not receive any. Outcome was determined as response to transplant, PFS and overall survival (OS) in months by Kaplan-Meier analysis using SPSS (IBM SPSS Statistics 21; IBM Corp., Chicago, IL) statistical tool kit.
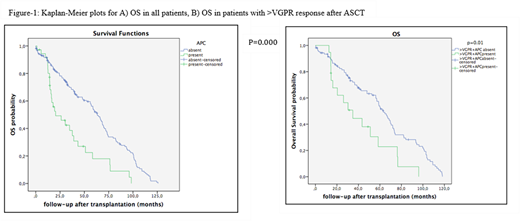
Results: A total of 209 patients with MM with routine assessment for APC in the apheresis product were included in the analysis. Of these patients, 195 who underwent ASCT, met the predetermined criteria for inclusion. There were 81 (41.5%) female and 114 (58.5%) male patients. The median age at diagnosis of MM was 56 years (range, 31-71 years). The interval from the time of diagnosis to ASCT was median 7 months and median follow-up from ASCT was 49 months (range, 3-198 months). Among 195 patients, 36 (18.5%) had evidence of APC contamination in the PBSC grafts ranging between 1x10-4-12.8 x10-4of total cells. Subtypes of MM were similar among those w/wo APC. Seventy-four patients had pre and post ASCT PET-CT imaging done with 28.2% still active lesions post-ASCT. There was no correlation between PET-CR and absence of APC in grafts. Neither was there a statistically significant association between disease response <VGPR at mobilization and the number of APC in the apheresis product. Post-transplant responses were closely associated with pre-transplant responses. A total of 25 and 57 patients relapsed/progressed in respectively 6 and 12 months after ASCT. Post-transplant response was significantly associated with relapse at 6 months (≥VGPR vs. <VGPR; 37.5% vs. 62.5%, p=0.000). Estimated median OS was significantly different between patients w/o APC contamination; 20.4±8.1 and 63.3±3.8, respectively (p=0.000) (Figure 1). There were no differences in OS among the patients achieving VGPR/CR at the time of mobilization and also two months after the ASCT. We performed a subgroup analysis of patients in grouping together VGPR/CR or only CR and among those patients presence of APC was correlated with worse OS (Figure 1). PFS at 2 years were 60.3±4.3% and 75.6±9.7% in patients receiving APC free PBSC grafts compared with grafts with evidence of APC contamination, respectively (p=0.595).
Discussion:PostASCT immune response was predictive for OS only when combined with APC results. Our study provides new insight suggesting a survival advantage value of residual APC detected by FCM, even at a low sensitivity level, on OS but not PFS following ASCT. The impact of such analysis may be more relevant on PFS with better assessment of residual APC with next generation flow cytometric/sequencing approaches while taking into account maintenance.
Ilhan:Roche: Speakers Bureau; BMS: Speakers Bureau; Alexion: Speakers Bureau; Celgene: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal